Are you searching for the difference between Liquid and Gas? You are at the correct place, we will guide in detail by our article.
There are three fundamental states of matter, i.e. solid, liquid and gas. Solids have a definite shape while liquid and gases diffuse to fill the available volume completely and do not have a definite shape.
Before moving directly to the difference between Liquid and Gas, it’s important to understand the terms individually.
Liquid
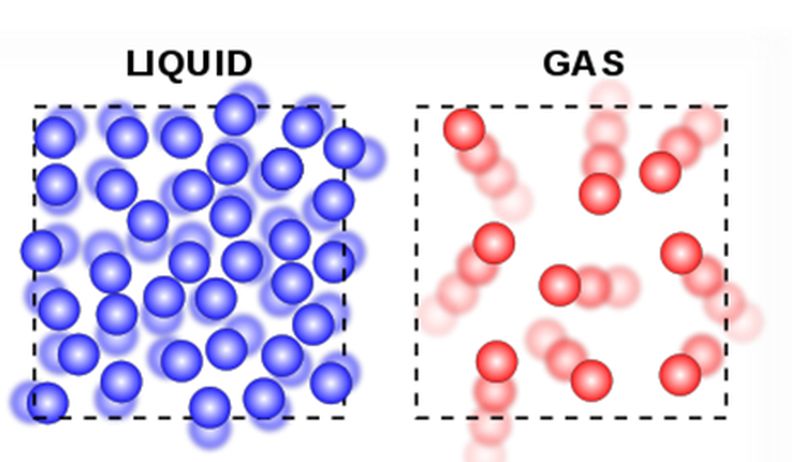
The liquid is an almost incompressible fluid which takes the shape of the container as it does not have a definite shape but retains a constant volume independent of pressure. The molecules of liquids have a moderate force of attraction. This results in the free movement to molecules within liquids.
Diffusion of liquids is higher than solids. The liquids do not have fixed shape but have fixed volume and have the ability to flow from higher to a lower level. Liquids are not hard but after frozen becomes hard.
A liquid is made up of atoms that are close, but not tightly bound to each other. Sound speed is faster than gaseous medium.
Gas
A gas is a state of matter that has neither definite shape nor a definite volume. The molecules in gases have a weak force of attraction between them and are very loosely packed and that’s why they take the shape of the container.
Gases can be easily compressed as the gases have a lot of space between the molecules which makes it spread around freely. Most of the gases are not easy to observe so; they are characterized by the volume, the number of particles, pressure, and temperature.
Gases need a closed container for storage. Sound speed is comparatively lower than liquid medium. Gases can flow in all the directions. A density of gases is low. Gases are not hard and can flow easily.
https://www.youtube.com/watch?v=9d1jK_2FMu8
Now take a view on the difference between Liquid and Gas for complete knowledge.
The fixed shape and Fixed volume
Liquids do have a definite shape but have definite volume while gases neither have fixed shape nor fixed volume.
Fluidity
Liquids have the ability to flow from higher to the lower level while gases can flow in all directions.
Density
Liquids have moderate density while gases have low density.
Interparticle space
The liquids have less intermolecular space than solids while gases have a lot of intermolecular space between them.
Storage
Liquids cannot be stored without containers and do not need closed container while gases need a closed container for storage.